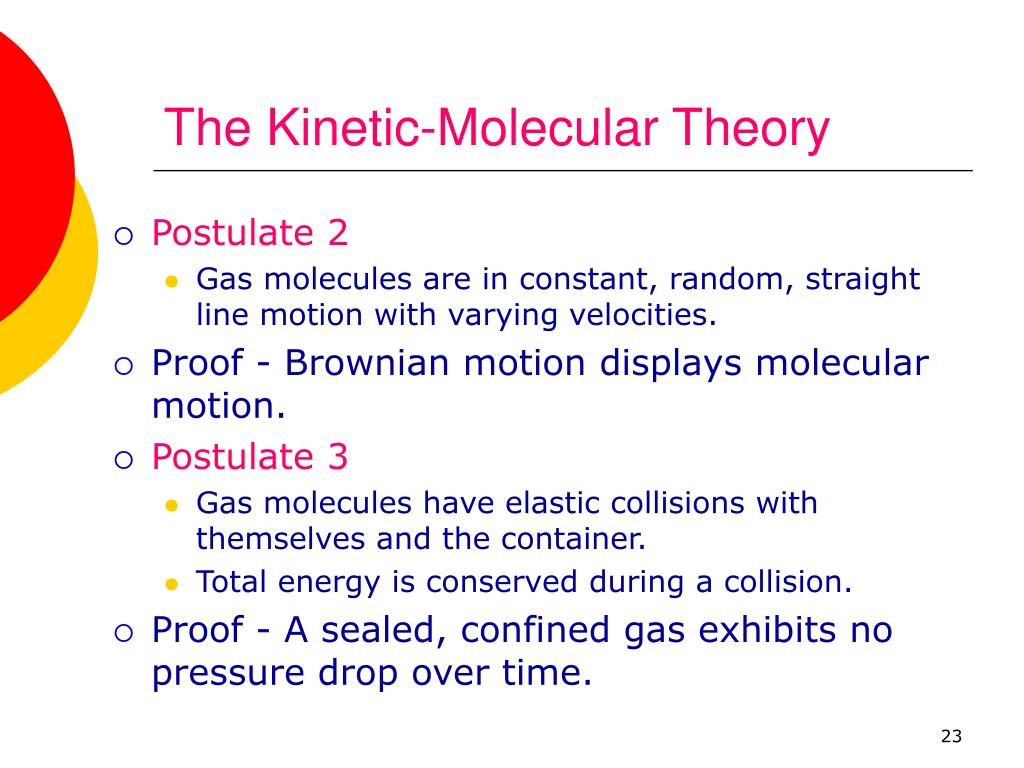
What Is The Kinetic Molecular Theory Of Heat . the motion of molecules in a gas is random in magnitude and direction for individual molecules, but a gas of many molecules. relate temperature to average kinetic energy. kinetic molecular theory is a theory that explains the behavior of a gas in terms of the motion and interactions of its constituent particles, which are typically atoms or molecules. Describe the behavior of an ideal gas. the experimental observations about the behavior of gases discussed so far can be explained with a simple theoretical model known as the kinetic. according to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to. Relate mass to molecular speed.
from www.slideserve.com
Describe the behavior of an ideal gas. Relate mass to molecular speed. kinetic molecular theory is a theory that explains the behavior of a gas in terms of the motion and interactions of its constituent particles, which are typically atoms or molecules. the experimental observations about the behavior of gases discussed so far can be explained with a simple theoretical model known as the kinetic. according to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to. the motion of molecules in a gas is random in magnitude and direction for individual molecules, but a gas of many molecules. relate temperature to average kinetic energy.
PPT CHAPTER 12 GASES AND THEORY PowerPoint
What Is The Kinetic Molecular Theory Of Heat kinetic molecular theory is a theory that explains the behavior of a gas in terms of the motion and interactions of its constituent particles, which are typically atoms or molecules. Describe the behavior of an ideal gas. Relate mass to molecular speed. the experimental observations about the behavior of gases discussed so far can be explained with a simple theoretical model known as the kinetic. according to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to. the motion of molecules in a gas is random in magnitude and direction for individual molecules, but a gas of many molecules. kinetic molecular theory is a theory that explains the behavior of a gas in terms of the motion and interactions of its constituent particles, which are typically atoms or molecules. relate temperature to average kinetic energy.
From www.slideserve.com
PPT Gases and the Theory PowerPoint Presentation What Is The Kinetic Molecular Theory Of Heat the experimental observations about the behavior of gases discussed so far can be explained with a simple theoretical model known as the kinetic. Describe the behavior of an ideal gas. relate temperature to average kinetic energy. according to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to. Relate mass to. What Is The Kinetic Molecular Theory Of Heat.
From karsonsrrichards.blogspot.com
Molecular Theory of Gases KarsonsrRichards What Is The Kinetic Molecular Theory Of Heat according to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to. relate temperature to average kinetic energy. Describe the behavior of an ideal gas. the motion of molecules in a gas is random in magnitude and direction for individual molecules, but a gas of many molecules. Relate mass to molecular. What Is The Kinetic Molecular Theory Of Heat.
From www.slideserve.com
PPT Heat & Thermodynamics PowerPoint Presentation, free download ID What Is The Kinetic Molecular Theory Of Heat the motion of molecules in a gas is random in magnitude and direction for individual molecules, but a gas of many molecules. Describe the behavior of an ideal gas. kinetic molecular theory is a theory that explains the behavior of a gas in terms of the motion and interactions of its constituent particles, which are typically atoms or. What Is The Kinetic Molecular Theory Of Heat.
From www.slideserve.com
PPT Theory PowerPoint Presentation, free download What Is The Kinetic Molecular Theory Of Heat Describe the behavior of an ideal gas. according to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to. Relate mass to molecular speed. the motion of molecules in a gas is random in magnitude and direction for individual molecules, but a gas of many molecules. the experimental observations about the. What Is The Kinetic Molecular Theory Of Heat.
From www.coursehero.com
Molecular Theory of Matter Boundless Chemistry Course Hero What Is The Kinetic Molecular Theory Of Heat Relate mass to molecular speed. the motion of molecules in a gas is random in magnitude and direction for individual molecules, but a gas of many molecules. Describe the behavior of an ideal gas. relate temperature to average kinetic energy. the experimental observations about the behavior of gases discussed so far can be explained with a simple. What Is The Kinetic Molecular Theory Of Heat.
From www.vrogue.co
Theory Of Heat vrogue.co What Is The Kinetic Molecular Theory Of Heat according to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to. the experimental observations about the behavior of gases discussed so far can be explained with a simple theoretical model known as the kinetic. relate temperature to average kinetic energy. Describe the behavior of an ideal gas. the motion. What Is The Kinetic Molecular Theory Of Heat.
From www.slideserve.com
PPT Chapter 10 Gases & the Atmosphere PowerPoint Presentation ID611285 What Is The Kinetic Molecular Theory Of Heat according to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to. relate temperature to average kinetic energy. kinetic molecular theory is a theory that explains the behavior of a gas in terms of the motion and interactions of its constituent particles, which are typically atoms or molecules. the motion. What Is The Kinetic Molecular Theory Of Heat.
From www.chemistrystudent.com
Collision Theory (ALevel) ChemistryStudent What Is The Kinetic Molecular Theory Of Heat the experimental observations about the behavior of gases discussed so far can be explained with a simple theoretical model known as the kinetic. relate temperature to average kinetic energy. Relate mass to molecular speed. the motion of molecules in a gas is random in magnitude and direction for individual molecules, but a gas of many molecules. . What Is The Kinetic Molecular Theory Of Heat.
From rumble.com
molecular theory, ideal gases Chemistry What Is The Kinetic Molecular Theory Of Heat according to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to. Describe the behavior of an ideal gas. the motion of molecules in a gas is random in magnitude and direction for individual molecules, but a gas of many molecules. relate temperature to average kinetic energy. the experimental observations. What Is The Kinetic Molecular Theory Of Heat.
From www.slideserve.com
PPT KMT PowerPoint Presentation, free download ID6901291 What Is The Kinetic Molecular Theory Of Heat relate temperature to average kinetic energy. according to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to. Relate mass to molecular speed. the experimental observations about the behavior of gases discussed so far can be explained with a simple theoretical model known as the kinetic. the motion of molecules. What Is The Kinetic Molecular Theory Of Heat.
From www.slideserve.com
PPT Molecular Theory (KMT) PowerPoint Presentation, free What Is The Kinetic Molecular Theory Of Heat kinetic molecular theory is a theory that explains the behavior of a gas in terms of the motion and interactions of its constituent particles, which are typically atoms or molecules. Describe the behavior of an ideal gas. the motion of molecules in a gas is random in magnitude and direction for individual molecules, but a gas of many. What Is The Kinetic Molecular Theory Of Heat.
From www.slideserve.com
PPT Thermodynamics PowerPoint Presentation, free download ID3521096 What Is The Kinetic Molecular Theory Of Heat relate temperature to average kinetic energy. the motion of molecules in a gas is random in magnitude and direction for individual molecules, but a gas of many molecules. Relate mass to molecular speed. according to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to. Describe the behavior of an ideal. What Is The Kinetic Molecular Theory Of Heat.
From slideplayer.com
Molecular Theory and Gases ppt download What Is The Kinetic Molecular Theory Of Heat relate temperature to average kinetic energy. according to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to. the experimental observations about the behavior of gases discussed so far can be explained with a simple theoretical model known as the kinetic. Describe the behavior of an ideal gas. Relate mass to. What Is The Kinetic Molecular Theory Of Heat.
From www.slideserve.com
PPT Chapter 9 PowerPoint Presentation, free download ID314166 What Is The Kinetic Molecular Theory Of Heat Describe the behavior of an ideal gas. kinetic molecular theory is a theory that explains the behavior of a gas in terms of the motion and interactions of its constituent particles, which are typically atoms or molecules. according to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to. the experimental. What Is The Kinetic Molecular Theory Of Heat.
From www.slideserve.com
PPT Molecular Theory (KMT) PowerPoint Presentation, free What Is The Kinetic Molecular Theory Of Heat Relate mass to molecular speed. the experimental observations about the behavior of gases discussed so far can be explained with a simple theoretical model known as the kinetic. Describe the behavior of an ideal gas. relate temperature to average kinetic energy. according to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly. What Is The Kinetic Molecular Theory Of Heat.
From www.slideserve.com
PPT Molecular Theory States of Matter Phase Changes What Is The Kinetic Molecular Theory Of Heat relate temperature to average kinetic energy. kinetic molecular theory is a theory that explains the behavior of a gas in terms of the motion and interactions of its constituent particles, which are typically atoms or molecules. according to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to. the motion. What Is The Kinetic Molecular Theory Of Heat.
From www.slideserve.com
PPT The Molecular Theory PowerPoint Presentation, free What Is The Kinetic Molecular Theory Of Heat the experimental observations about the behavior of gases discussed so far can be explained with a simple theoretical model known as the kinetic. kinetic molecular theory is a theory that explains the behavior of a gas in terms of the motion and interactions of its constituent particles, which are typically atoms or molecules. Relate mass to molecular speed.. What Is The Kinetic Molecular Theory Of Heat.
From slidetodoc.com
Thermodynamics Theory of Heat Molecules in motion What Is The Kinetic Molecular Theory Of Heat relate temperature to average kinetic energy. Relate mass to molecular speed. Describe the behavior of an ideal gas. kinetic molecular theory is a theory that explains the behavior of a gas in terms of the motion and interactions of its constituent particles, which are typically atoms or molecules. the motion of molecules in a gas is random. What Is The Kinetic Molecular Theory Of Heat.